CUSTOMER SERVICE
SpineMED® Innovative Medizintechnik GmbH relies on a strong and exclusive partnership with SLE Germany GmbH for customer service. The company, which has been in operation since 2001 with more than 20 highly qualified employees, handles over 5000 field service and in-house service assignments per year. S-L-E Germany GmbH has thus been one of the leading providers of technical services in the fields of hospital technology, device management and technical service in many parts of the world of medical technology for over 15 years. As part of the S-L-E Medical Group, the company operates internationally and offers its services with S-L-E Nordic AB in all Scandinavian countries. S-L-E Germany GmbH handles the complete technical service and support of SpineMED® devices. As a certified company, S-L-E Germany GmbH operates strictly according to DIN EN ISO 13485:2012 and has a corresponding quality management system.
QUALITY
Based on years of clinical experience and feedback from healthcare professionals, the SpineMED® system was developed in Canada to overcome the limitations and undesirable side effects of previous generations of devices. Cumbersome nylon straps, outdated tower designs, and obsolete traction components have been eliminated and replaced with a patented restraint system, an exclusive pelvic tilt mechanism, and advanced computer controls.
Quality and innovation are cornerstones of the SpineMED® philosophy. The manufacturer of SpineMED® devices, Spinemed Inc., 1648 South Ogilvie Street, Prince George, British Columbia V2N 1W9, is an ISO13485 and CE certified manufacturer of medical devices and holds numerous patents on the unique design and key components of SpineMED®. The result is a decompression system that is manufactured to global hospital standards and whose quality is unparalleled in the industry.
The SpineMED® decompression system has been developed and certified to global medical standards. FDA 510(k) #k05103, CE 2862, TUV, ISO 13485, Health Canada Medical Device License #67289

and disc patients
SpineMED® OFFERS A VERY WIDE
RANGE OF APPLICATIONS
The SpineMED® procedure is suitable for patients with disc bulging/prolapse, sciatica, radicular and pseudoradicular symptoms, spinal canal stenosis (non-osseous) and disc degeneration.
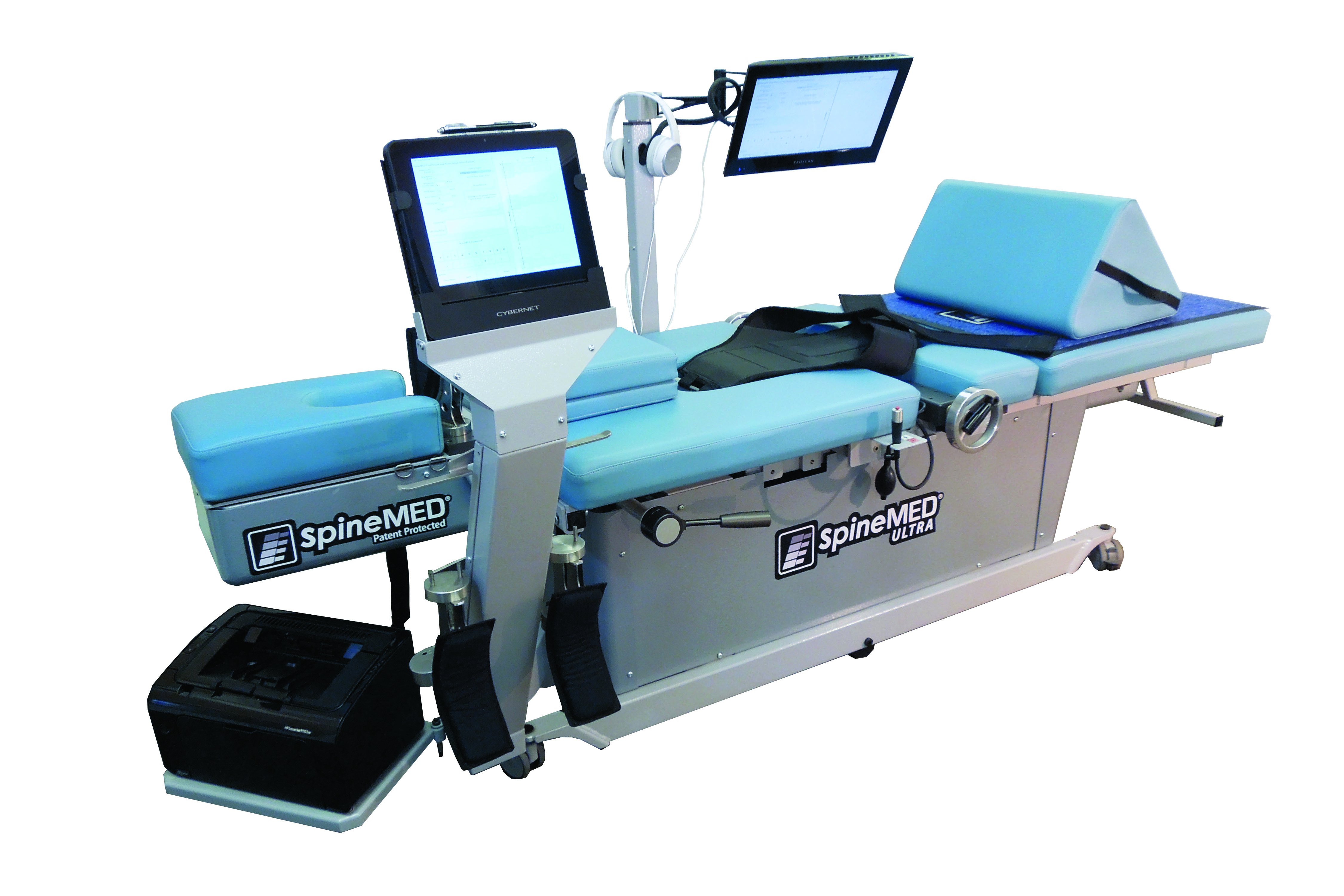
SpineMED®, the world leader in non-surgical spinal decompression, is a system designed to relieve (decompress) damaged and degeneratively altered intervertebral discs of a specific spinal segment by means of distraction and positioning.
Pathological disc changes are among the most common causes of pain in the cervical and/or lumbar spine. A strong, often one-sided load on the spine, in conjunction with lack of exercise, poor posture and excess weight, leads to degeneration of the intervertebral disc. The result is dehydration of the intervertebral disc and, consequently, a reduction in the intervertebral space. This in turn promotes facet joint syndromes, stenoses (especially lateral foramen) and disc protrusions/prolapses, as injuries and tears occur in the area of the annulus due to the intradiscal pressure increase. Disc herniations, in turn, can lead to radiculopathies with both sensory and motor deficits.
Damaged and injured intervertebral discs heal very slowly – if at all – as they are still exposed to a permanent pressure load. In addition to any parallel drug and anti-inflammatory therapy, the therapy of choice should be decompression and pressure relief of the intervertebral disc in order to stimulate the process of diffusion of fluid, oxygen and nutrients from the surrounding area (especially via the hyaline endplates of the adjacent vertebral bodies). At the same time, this stimulates the body’s natural healing process.
In addition, both rehydration and decompression promote a shift of the nucleus pulposus towards the center of the intervertebral disc, which reduces the process of space occupation and reduces any symptoms.
SAFE, EFFICIENT & SUCCESSFUL
SpineMED® performs a biofeedback-controlled treatment independently and computer-monitored. This makes the treatment very pleasant and painless. The biofeedback system allows for the use of low tensile forces. SpineMED® is a delegable and automated therapy!
Easy to implement
into practice
delegable
proven
many times
scientifically
proven
popular with patients
effective
No reflex muscle tension in the patient as with conventional traction treatments, because SpineMED® regulates the distraction forces computer-controlled every 20 milliseconds, whereby the natural muscular reflex mechanism is switched off.
Direct fixation of the patient to the pelvis or base of the skull (i.e. perfect set-up). This enables safe, accurate and repeatable distraction.
Precision through state-of-the-art technology. The patented SpineMED® procedure ensures exact positioning of the patient and optimal isolation of the desired spinal segment. This allows the decompression treatment to be localized very precisely and at the same time optimally dosed. This works with relatively low distraction forces.
Advantage through know-how. The SpineMED® Table is a medical device certified in Germany and in the European Union, has a CE mark and is subject to worldwide medical device directives. Both technology and design are patented and the software is protected by copyright.
CONTACT US!
You are currently viewing a placeholder content from HubSpot. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information